40 cautionary and advisory labels for medicines
Cautionary and advisory labels for medicines - Everything2.com These are the code numbers and their meanings for the cautionary labels used by pharmacist s when dispensing medicine s in the UK. Extra counselling may be given with relation to age, experience , background and understanding of the patient. Warning. May cause drowsiness What auxiliary label to use for Zithromax - Drugs.com SU. suzanne66 15 Dec 2009. Zithromax tablets cautionary and advisory labels include numbers 5, 9 & 23. 5. Do not take indigestion remedies at the same time of day as this medicine. 9. Take at regular intervals. Complete the prescribed course unless otherwise directed. 23.
Labels | About | BNF content published by NICE Label 12 should not be used for anticoagulants since label 10 is more appropriate. 13 Dissolve or mix with water before taking To be used on preparations that are intended to be dissolved in water (e.g. soluble tablets) or mixed with water (e.g. powders, granules) before use. In a few cases other liquids such as fruit juice or milk may be used. 14

Cautionary and advisory labels for medicines
Required Advisory Statements for Medicine Labels (RASML ... Australian labelling requirements for non-prescription medicines (Therapeutic Goods Order No. 92) require some over-the-counter and complementary medicine labels to contain particular warning statements ('advisory statements') about specific risks related to use of the medicines. › en › health-canadaGuidance Document: Labelling of Pharmaceutical Drugs ... - Canada Nov 01, 2013 · The purpose of this document is to provide guidance to sponsors to facilitate compliance with the labelling requirements pursuant to sections 3, 9, and 10 of the Food and Drugs Act as well as related provisions of the Food and Drug Regulations, the Controlled Drugs and Substances Act, and its related Regulations including the Narcotic Control Regulations, Parts G and J of the Food and Drug ... Safer dispensing labels for prescription medicines ... In a US trial, 395 participants were given five common prescription medicines with a dispensing label and asked how they would take the medicine. 4 The medicines and their instructions included: amoxicillin, 'take one teaspoonful by mouth three times a day' furosemide (frusemide), 'take one tablet in the morning and one at 5 pm'
Cautionary and advisory labels for medicines. Cautionary_and_advisory_label - chemeurope.com Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counselling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Label Wording and Warnings Cautionary And Advisory Labels - Cautionary And Advisory ... Cautionary And Advisory Labels. These labels are available in eye-catching fluro orange with different statements or instructions. We recommend using these labels to assist care facilities to maximise the safety by affixing appropriate labels to the pack where necessary. Labels measure 40mm x 20mm. Description. PDF Revisions to APF24 Cautionary advisory labels Revisions to Table A2. Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Abacavir Oral solution: 7b (60 days), 12†, 21 Tablet: 12†, 21, A Aciclovir Eye ointment: 7b (28 ... Cautionary and Advisory Labels - Bagot Press PHARMACEUTICAL > Cautionary and Advisory Labels The Bagot Press Cautionary and Advisory label system is one of the few approved licences used for printing labels for prescribed medicines and the bright colour labels draw attention to important specific information to patients. Ordering Guide Can't find what you are looking for? Contact us
Cautionary Advisory Labels (CAL) - Medi Print Cautionary Advisory Labels (CAL) Laser Labels Drug Labels Medical Filing Labels Medical Alert Labels Health Professional Labels X-Ray Labels Pharmacy Labels Other Labels & Accessories GHS - Global Harmonized System Labels Guidance for cautionary and advisory labels | About | BNF ... Wordings which can be given as separate warnings are labels 1-19, 29-30, and 32. Wordings which can be incorporated in an appropriate position in the directions for dosage or administration are labels 21-28. A label has been omitted for number 20; labels 31 and 33 no longer apply to any medicines in the BNF and have therefore been deleted. PDF Required Advisory Statements for Medicine Labels updates ... The TGA is in the process of revising and updating the Required Advisory Statements for Medicine Labels (Edition 1, including update 4) dated September 2008 (RASML). The proposed changes were made in consultation with a working group consisting of the Office of Complementary Medicines and OTC Medicines Evaluation section. Label Statements Database - Medsafe This database lists the warning and advisory statements that are required on medicine and related product labels under regulations 13 (1) (i) and 14 (1) (f) of the Medicines Regulations 1984. Words of a similar meaning to the statements in the database may be used and individual statements may be combined provided the intent is not changed.
New Label 24 to help pharmacists reduce opioid risks ... New Label 24 to help pharmacists reduce opioid risks. In line with Therapeutic Goods Administration (TGA) regulatory changes to enhance medicine safety, PSA has developed a cautionary advisory label (CAL) that warns consumers about the risk of opioid overdose and dependence. The CAL (Label 24, at right) can be applied to opioid medicines at the ... 50 Common Warning Labels On Medication Containers ... Top 50 Common Warning Labels and Their Meanings. The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even ... PROMETHAZINE HYDROCHLORIDE | Medicinal forms | BNFc ... Cautionary and advisory labels. Label 2 - Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol . Phenergan 10mg tablets (Sanofi) Active ingredients Size Unit NHS indicative price Drug tariff ... Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication.
TRAMADOL WITH PARACETAMOL | Medicinal forms | BNFc content ... Cautionary and advisory labels. Label 2 - Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Do not drink alcohol . Label 25 - Swallow this medicine whole. Do not chew or crush . Label 29 - Do not take more than 2 at any one time. Do not take more than 8 in 24 hours
Cautionary and advisory labels for medicines - SlideShare Cautionary and advisory labels for medicines 1. CAUTIONARY AND ADVISORY LABELS (CALS) B y Kiran Sharma KIET School of Pharmacy 2. CALs Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient.
Cautionary Advisory Labels (CAL) - Medi Print This medicine may cause dizziness - CAL. This medicine may cause dizziness - CAL Code: CAL-16 Size: 11 x 40mm Quantity: 1,000 per ro.. Add to Cart.

A selection of warning labels is available at a Southern California hospital pharmacy to ensure ...
StirlingFildes | HealthCare Cautionary Advisory Labels. The StirlingFildes Cautionary Advisory Label (CALs) system produced by the PSA is a valuable. labelling system designed for helping pharmacists to fulfil their legal and professional. obligations, promote quality use of medicines, and provide optimal outcomes for consumers. All labels are supplied in a convenient ...
cautionary and advisory label : definition of cautionary ... Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels.
Cautionary and advisory labels for dispensed medicines Cautionary and advisory labels for dispensed medicines Numbers following the preparation entries in the BNF correspond to the code numbers of the cautionary labels that pharmacist are recommended to add when dispensing. It is also expected that pharmacists will counsel patients when necessary.
Medicine labels - what do the warning statements mean ... Some medicines can cause stomach problems, indigestion and problems with your throat and oesophagus (the pipe between your throat and stomach) if they are not swallowed properly. Taking them with a large glass of water helps to wash them down. Take each dose on an empty stomach - one hour before or two hours after food.
StirlingFildes | Printing, Packaging and Consumables Pharmacy Labelling Printed Pharmacy Bags Printed Stationery Items Generic Bags & Packaging Promotional Products General Stationery Items POS Consumables & Price Marking Printers & Toners Deblistering Gift Wrap & Bags Cautionary & Advisory Labels Buy a mixture of 12 or more labels and save. The savings will be calculated on your invoice.
› Details › F2019C00136Therapeutic Goods Regulations 1990 - Legislation Feb 12, 2019 · Required Advisory Statements for Medicine Labels means the advisory statements specified by the Minister by legislative instrument under subsection 3(5A) of the Act. sample includes part of a sample. serious, in relation to a form of a disease, condition, ailment or defect, means a form of the disease, condition, ailment or defect that is:
Cautionary advisory labels - Australian Pharmacist The dispensing pharmacist has dispensed the patient's discharge medications and has labelled simvastatin with cautionary advisory label 21 (Special handling and disposal required - ask your pharmacist) and label A (Swallow whole do not crush or chew). You are concerned that these labels may alarm and/or confuse the patient. What should you do?
PDF Revisions to APF25 Cautionary advisory labels Cautionary advisory labels Revisions to Table 2.2 Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Acetazolamide 8, 10a, 12 Adalimumab 6 (except syringe in use), 7b*, 13
PDF cautionary advisory labels - Openbook Howden Print & Design cautionary advisory labels OBH 18642 CAL's are a valuable tool for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers. Reproduced with the permission of the Pharmaceutical Society of Australia. Sold in dispenser boxes of 1000.
Cautionary Advisory Labels Flashcards | Quizlet Cautionary Advisory Labels. STUDY. PLAY. Diazepam. o Label 1: This medicine may cause DROWSINESSS and may increase effects of alcohol. If affected, do not drive a motor vehicle or operate machinery o Label 1a: This preparation is to aid sleep. Drowsiness may continue the following day. If affected, do not drive or operate machinery.
Safer dispensing labels for prescription medicines ... In a US trial, 395 participants were given five common prescription medicines with a dispensing label and asked how they would take the medicine. 4 The medicines and their instructions included: amoxicillin, 'take one teaspoonful by mouth three times a day' furosemide (frusemide), 'take one tablet in the morning and one at 5 pm'

The Importance of Reading Medication Warning Labels | Blog Community | Medical and Health Care ...
› en › health-canadaGuidance Document: Labelling of Pharmaceutical Drugs ... - Canada Nov 01, 2013 · The purpose of this document is to provide guidance to sponsors to facilitate compliance with the labelling requirements pursuant to sections 3, 9, and 10 of the Food and Drugs Act as well as related provisions of the Food and Drug Regulations, the Controlled Drugs and Substances Act, and its related Regulations including the Narcotic Control Regulations, Parts G and J of the Food and Drug ...

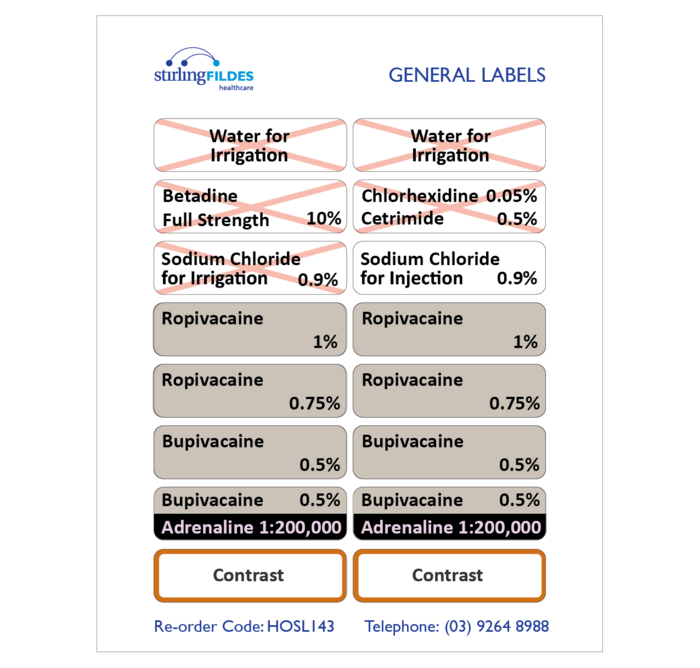



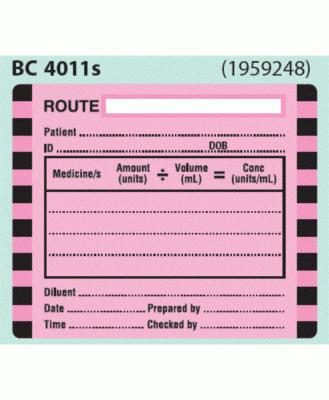

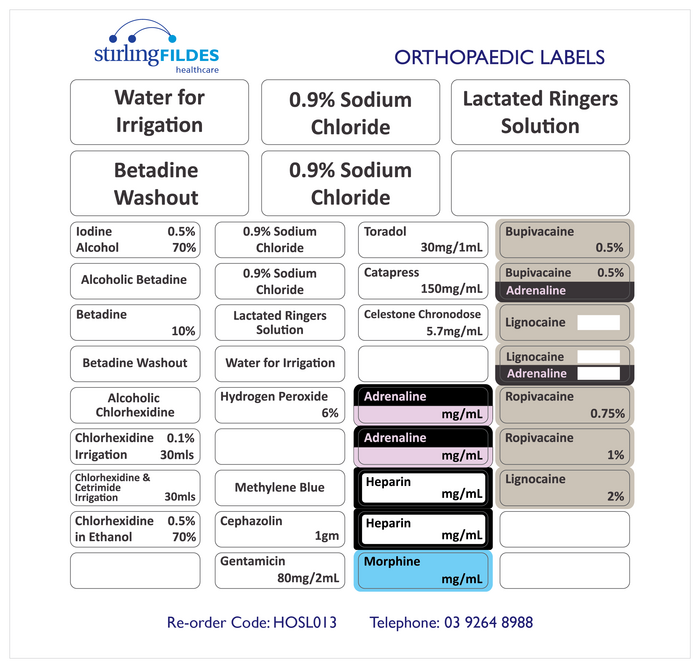



Post a Comment for "40 cautionary and advisory labels for medicines"